Researchers at the University of Alabama at Birmingham (UAB) have announced a breakthrough treatment that slows the progression of Huntington’s disease by 75%, a first in the fight against this devastating disorder.
The new gene therapy, known as AMT-130, is part of a small clinical trial led in collaboration with uniQure.
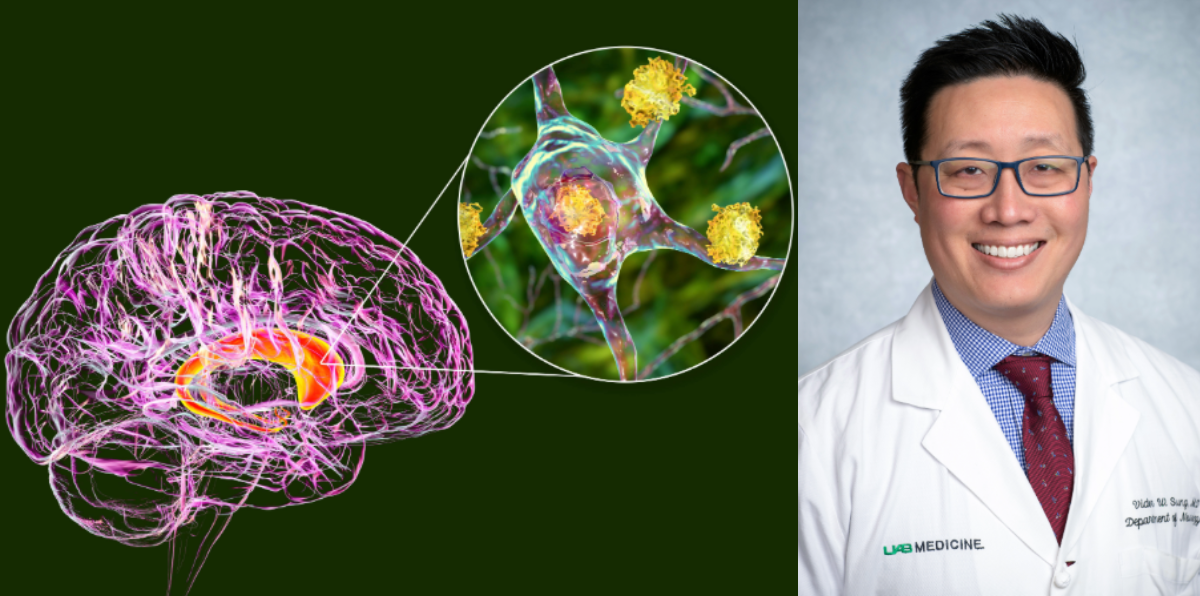
Seven of the 17 high-dose participants were treated at UAB, where neurologist Dr. Victor Sung, director of the Division of Movement Disorders and the UAB Huntington’s Disease Clinic, called the results “a huge step” toward changing the course of a disease long considered untreatable.
“Since the HD gene was first sequenced over 30 years ago, we have been on a quest to try to slow or stop this difficult disease, and these preliminary results are finally a huge step in that direction,” Sung said.
Until now, treatments for Huntington’s disease have only managed symptoms rather than altering its course. The UAB-led research marks the first time a therapy has successfully slowed disease progression, offering hope for patients who have long been told there was little doctors could do to change their prognosis.
The study tested low and high doses of AMT-130 in 29 participants diagnosed in the early stages of Huntington’s disease (HD).
According to UAB, the treatment, delivered through an eight to ten-hour brain surgery, works by blocking messenger RNA (mRNA) responsible for producing the toxic huntingtin protein — the culprit behind neuronal damage in HD.
Unlike some genetic therapies, AMT-130 does not alter DNA, providing a significant safety advantage.
Patients receiving the high-dose treatment showed 75 percent slower disease progression over three years compared to those who did not receive the therapy. They also maintained better movement, cognitive function, and daily living abilities, with no significant side effects reported.
While invasive, the therapy is designed as a single-dose treatment that could last a lifetime, potentially offering a “one and done” solution. Because it does not permanently alter DNA, it also avoids many of the risks associated with gene editing.
“We certainly remain cautious about interpretation of these results since they are only in a small number of patients, but the 75 percent disease-slowing is a big number and still very much a reason to be excited,” Sung said.
The treatment has not yet received FDA approval, but uniQure is seeking accelerated authorization. If approved, AMT-130 would become the first effective disease-modifying therapy for Huntington’s disease.
Dr. Sung acknowledged that challenges remain in making the treatment widely accessible, both surgically and financially, but emphasized that these are “far preferable to continuing without any effective therapies that can alter the course of Huntington’s disease.”
He also praised the bravery of participants who volunteered for the trial. “I also think it is important to highlight the bravery of these 29 patients, a significant proportion of whom were our patients here at UAB, who underwent surgery to have a drug with permanent effects injected into the brain before we even knew if it was safe,” Sung said. “But if their courage leads to the first FDA-approved disease-modifying therapy in HD, what a legacy that will be, and how exciting that UAB will have been such a big part of that.”
Additional information on the clinical trial is available on the UAB website.
According to UCSF Health, Huntington’s disease (HD) is a fatal hereditary condition marked by involuntary movements, cognitive decline, and emotional disturbances. It is caused by genetically programmed degeneration of neurons — brain cells — in specific areas of the brain. This degeneration leads to uncontrolled movements, loss of intellectual ability, and emotional instability.
Huntington’s disease is inherited through a gene mutation passed from parent to child. Each child of an affected parent has a 50 percent chance of inheriting the mutation. If the gene is not inherited, the child will not develop HD and cannot pass it on. If the gene is inherited, the child will eventually develop the disease. Whether one sibling inherits the gene has no effect on whether others do, and 1 to 3 percent of cases occur without any family history due to new genetic mutations.
In the United States, Huntington’s disease affects one in every 10,000 to 20,000 people, impacting men and women equally across all racial and ethnic backgrounds. Symptoms typically appear between the ages of 30 and 55.
Sherri Blevins is a staff writer for Yellowhammer News. You may contact her at [email protected].