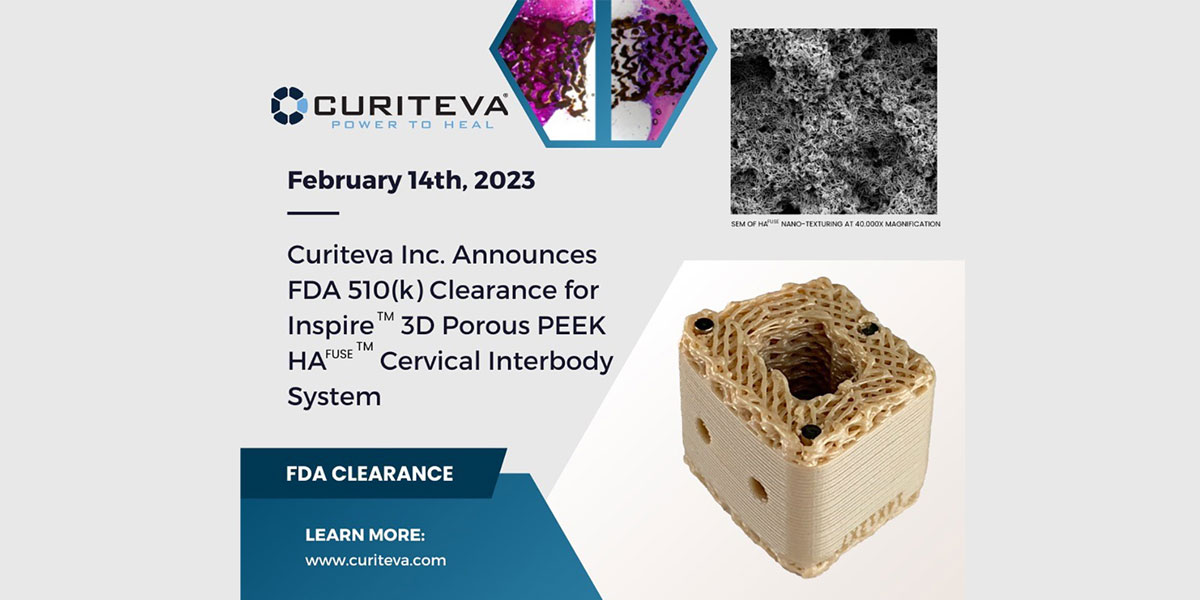
TANNER – Two years ago, medical device manufacturer Curiteva, designed, programmed and built a first-of-its-kind 3D printer that uses a popular thermoplastic polymer known as PEEK, to make implantable medical devices for the spinal orthopedic industry.
This week, after 16 months of waiting, Curiteva received regulatory clearance from the Food and Drug Administration for implantation of those devices in humans, specifically for cervical spine surgery.
“By building the PEEK printer, Curiteva did something that has never been done. We built our own manufacturing machine,” said Curiteva CEO Mike English. “Now we are the first technology company in the United States, and we think in the world, to get regulatory body clearance from the FDA for an implantable medical device printed out of PEEK.”
English said FDA510(k) essentially lets a company use a predicate pathway to establishing a new device or implant, as long as the company can show an equivalence to a similar device already on the market, but different in some way.
“That process usually takes 90 days, but ours took much longer because our technology was novel and new to the FDA,” said English.
Curiteva’s 35,000-square-foot production facility in Tanner was a traditional manufacturing company until 2017, according to English. That was when they started making medical devices they could sell in the commercial marketplace.
In late 2020, Curiteva acquired the technology for a 3D printing process for Polyetheretherketone. Better known as PEEK, English said it has been a gamechanger in 3D manufacturing, but it is now taking off in orthopedics and other healthcare applications.
“Titanium alloy has been the method for printing medical devices for the last decade,” English said. “3D is being used in a lot of healthcare applications, but it is all done with polymers for prototyping, surgical planning, and things like. But not for something that goes into the body.
“In less than 2.5 years, the PEEK printer we built went from a cool invention, to a FDA-approved commercial medical device that allows us to sell implant devices to neurosurgeons and orthopedic surgeons all over the U.S.”
He said the company plans a commercial launch in key academic centers across the U.S.
“Curiteva is uniquely positioned to control the product development process of traditional implants and 3D-printed devices from inception to commercialization and scale to meet market demand.”